 If you are a beginner to brewing you can skip this article completely and come back to it at a later stage in your apprenticeship. If you are brewing from kits there is no need to worry about water treatment at all. If you are brewing from malt extract there is still probably no need to concern yourself with the matter. The main benefits of water treatment are obtained when mashing from grain, but even so, perfectly acceptable ales can still be brewed without it. Only if you are a perfectionist and an experienced brewer seeking to fine-tune the quality of your beers should you need to concern yourself with water treatment.
If you are a beginner to brewing you can skip this article completely and come back to it at a later stage in your apprenticeship. If you are brewing from kits there is no need to worry about water treatment at all. If you are brewing from malt extract there is still probably no need to concern yourself with the matter. The main benefits of water treatment are obtained when mashing from grain, but even so, perfectly acceptable ales can still be brewed without it. Only if you are a perfectionist and an experienced brewer seeking to fine-tune the quality of your beers should you need to concern yourself with water treatment.
Read also:
Ingredients for brewing: grain, sugar, malt extract
Traditional Commercial Brewing
Home Brewing. Beginners Start Here
Home Brewing. What should You Know about the Hops
All You should Know about Brewery Yeast
Home Brewing. Mashing and Sparging
The water should be soft by all means. That of brooks and rivers is best. That of a pond, fed by a rivulet, or spring, will do very well. Rainwater, if just fallen, may do; but stale rain-water, or stagnant pond-water, makes the beer flat and difficult to keep; and hard water, from wells, is very bad it does not get the sweetness out of the malt, nor the bitterness out of the hops, like soft water; and the wort of it does not ferment well, which is certain proof of its unfitness for the purpose.
William Cobbett, Cottage Economy, 1821
Liquid assets
The mineral content of brewing water is an important factor in the brewing process, but the suitability of various water supplies for brewing has been the subject of much myth and misconception over the years. The truth is that water treatment is neither as complex nor as important as a lot of people seem to think. That is not to say that the subject should be completely ignored, but it is certainly not worth losing any sleep over. The great London porter brewers of old did not build their breweries in London because the water suited them. They built their breweries in London because that was where the people happened to be. Burton upon Trent became a brewing town only because a bunch of Benedictine monks who knew how to brew happened to live there. Centuries of experience, helped by the River Trent, the Trent and Mersey Canal, and the Midland Railway, did the rest for the great Burton brewers. There are breweries loacated all over the country who have been making perfectly satisfactory ales for two hundred years or more - long before water treatment was ever thought of. There is no domestic water supply in Britain that will make bad beer!
Sources of water
All our water comes to us in the form of rain. As it falls to earth it picks up quantities of atmospheric gases and pollutants which acidify it slightly. The most significant of these acids being carbonic acid which is produced by the admission of atmospheric carbon dioxide into solution.
This acidified rainwater falls to earth, drains through the topsoil, percolates through mineral substrates and porous rock below the soil, through crack, and fissures in non porous rock, and finally settles on to a table of impervious rock where it waits to be collected. Alternatively it may overflow into a river, or surface as a spring. On its journey through the earth the water absorbs mineral salts. The type and quantity of these salts absorbed is dependant upon the type of rocks and terrain through which the water passes before being collected.
Rainwater which falls on to sedimentary rocks will absorb minerals on its journey through the earth. Some of these minerals are directly soluble in water, whereas others need to react with the carbonic acid collected from the atmosphere to cause a chemical change in order to affect solubility.
Rainwater which falls on to insoluble rock such as slate or granite does not have the opportunity to pick up mineral salts and remains more or less mineral free. This water we know as soft water.
Only a few of the minerals found in water are important as far as brewing is concerned. The most important minerals are:
Calcium Bicarbonate now usually called calcium hydrogen carbonate, is the principal substance causing temporary hardness in our water supplies and is the one mineral that is undesirable in beer. It reacts adversely with the components of the mash, increasing its pH, which is the opposite effect to that desired under normal circumstances. Calcium bicarbonate is derived from calcium carbonate (common chalk). Calcium carbonate is insoluble in water, but in regions where there is a large proportion of calcium carbonate in the substrata, the carbonic acid in the rainwater reacts chemically with the carbonates to produce calcium bicarbonate which is soluble in water.
The presence of bicarbonate ions in our water has a detrimental effect on the quality of our ale. Bicarbonate ions interfere with the fermentation process, buffer and reduce the effect of the more important sulphate ions, and mess up fining systems. Most commercial breweries take steps to ensure that their bicarbonate hardness is reduced to less than 20 ppm.
Bicarbonate hardness is termed temporary hardness because it can be reduced by boiling the water, whereby it is broken down into carbon dioxide, water, and calcium carbonate (common chalk). The carbon dioxide is given off to the atmosphere and the chalk, being insoluble in water, settles out on the bottom of the boiler.
Calcium Sulphate is another of the principle substances causing hardness in some water supplies and is a very beneficial mineral as far as brewing is concerned, which is fortunate because it is difficult to remove. Calcium sulphate hardness is termed permanent hardness because it is not thrown out of solution upon tolling. Calcium sulphate reacts with other components in the mash, causing phosphates to precipitate, which releases phosphoric acid and therefore acidifies the won (lowers its pH). All the brewing processes benefit from the presence of calcium sulphate and a low mash pH. Keeping qualities are improved, clarity is improved, trub formation is improved, hop extraction is improved, astringent flavours are buffered, and yeast is more vigorous.
Calcium sulphate acts as a buffer and tends to suppress harshness in an ale. This means that a high level of hops can be used to provide a full hop flavour and aroma, without extracting an undesirable harshness from other resins contained in the hops. The presence of calcium sulphate also reduces the solubility of undesirable carbonates in water, and calcium ions are required by certain enzymes during mashing. However, if used to excess it can impart a harsh bitter taste to the ale. A further detrimental effect of using it in too high a quantity is the risk of precipitating polypeptides, polyphenols, hop resins, and too much phosphate out of solution. Calcium sulphate is commonly known as Gypsum.
Magnesium Sulphate in one mineral that sets the water of Burton upon Trent apart from the rest of the country. The water of Burton upon Trent has a relatively high level of magnesium sulphate when compared with the water of other areas. Magnesium is an important mineral required by the yeast during alcoholic fermentation, and magnesium sulphate provides the mineral in a permanent form, ie it will not precipitate out of solution upon boiling. Magnesium sulphate is said to have beneficial effects upon wort stabilisation during the boiling phase of our ale production. It does not have the same effect upon reducing the mash pH as does calcium sulphate and cannot be used for wort pH adjustment. Magnesium sulphate is more commonly known as Epsom salts, well known for providing inner cleanliness.
Sodium Chloride, common salt. London water contains a relatively high proportion of sodium chloride (common salt). London was famous for its porters and stouts. Because of this, many brewers claim that a high proportion of sodium chloride is necessary to produce dark ales, mild ales and stouts. There is no major scientific gain to be obtained by adding salt to brewing water. Although yeast does requires chloride ions to be present, there will be sufficient supplied by the ingredients. Therefore the benefit of sodium chloride is mostly flavour-related, for much the same reason that salt is added to vegetables when cooking. It acts as a flavour enhancer, smooths out harsh flavours, and contributes fullness and mouthfeel to the ale.
However, it is not absolutely necessary to add the salt directly to the water, it could be added to the mash, the boil, or you could probably add it your pint pot prior to drinking! The quantity employed is a matter of taste, but should not be sufficient to impart a distinct salt taste.
Why treat our water?
The primary reason for water treatment is to ensure that the pH of the mash is correct. Optimum mash efficiency is obtained at about pH 5.3, but, in general, the lower the mash pH the better. However, the mash will work perfectly well over a wide range of pH, so water treatment is not essential. It is also possible to control the fermentability of the wort by adjusting mash pH.
A low mash pH will produce a drier beer than a high mash pH, so water treatment offers a small degree of control for the experimentally minded. Furthermore, although our water authorities are obliged to give us water of good drinking quality, they are not obliged to give us water of good brewing quality! Modern water supplies contain added chlorine or fluorides, plus increasing levels of stray synthetic chemicals, the by-products of fertilisers and pesticides. Even the birth pill hormone can be detected in London water supplies!
Although water treatment is not strictly necessary, most ales can be improved by treating the water, although the only treatment necessary may be limited to boiling the water prior to brewing. I am of the opinion that all water used for brewing should at least be boiled to drive off purification chlorine/fluorine, which could taint the ale. Boiling also has the advantage that a high degree of sterility is ensured and that unwanted calcium bicarbonate is removed.
Burtonising
Originally, brewing water was treated in order to make its imposition similar to that of Burton upon Trent, a process known as Burtonising. In the old days breweries tried to imitate the water of Burton upon Trent in the misguided belief that this would help themimitate the superior Burton pale ales. In a more modern sense all forms of brewing water treatment are known as Burtonising.
Although the table below purports to show the mineral content of a typical Burton water, there are, in fact, several types of water beneath Burton upon Trent and there are several instances where two wells sunk a few feet apart provide water of two completely different mineral contents.
Table 1
Mineral content of a typical Burton water in ppm (mg/l)
The table 2 shows the additions required to 1 litre of SOFT water to achieve the figures given in table 1:
Table 2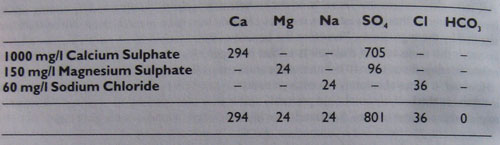
In hard water areas there will already be minerals present in the water, so indiscriminately adding more minerals to it is not likely to produce a water appropriate to Burton upon Trent. In fact, it could move it further away from the ideal. The only reliable way of imitating the water of Burton upon Trent is by chemical analysis of the available water supply and by modifying the water by adding or subtracting minerals accordingly. This is outside the scope of the average amateur brewer, but fortunately it does not matter. There is a far easier method.
Practical water treatment
Fortunately, in these more modern times, we know that it is not necessary for us to try to exactly imitate the water of Burton upon Trent. This was done in the days when no one knew why Burton brewers could produce better pale ale than could the London brewers. Nowadays water treatment consists of removing as much of the carbonate content of the water as possible and adding sulphates to lower the mash pH, or to bring the mash pH closer to the desired value.
Removal of calcium bicarbonate
No ale benefits from having carbonates or bicarbonates present. In hard water areas the first task in any water treatment procedure is the removal of excessive calcium bicarbonate. Calcium bicarbonate can be reduced to calcium carbonate by boiling and this is the most popular method as far as home brewing is conserned. This simply entails boiling the brewing water for about fifteen minutes, and then allowing it to stand for a while to enable the insoluble calcium carbonate to settle out. The water is then carefully transferred to a secondary container leaving the precipitate behind.
Removing this bicarbonate by boiling is more efficiently done if the water contains plenty of dissolved air prior to the boiling operation, a high level of dissolved oxygen helps drive off the carbon dioxide evolved in the process and prevent it recombining. As it happens, fresh mains water from your kitchen tap is probably rich in dissolved air, particularly if it is dispensed via one of those nozzles with a piece of gauze or perforated plastic inside which causes the water to foam. On the other hand, the act of filling your boiler will probably admit plenty of air. If you have any doubts about the matter, any sort of spraying action, or merely sloshing your water from one container to another with plenty of mechanical action will admit copious amounts of air. Of course, if you live in an area where the water is not 'chalky', you need not concern your-self with the matter.
The addition of acids to the water, such as citric acid or lactic acid, will also cause the precipitation of carbonates However, these acids are best added after the boiling period has elapsed. By adding the acid after boiling, the level of bicarbonate has already been reduced considerably and a more efficient end-result is achieved. The use of these acids to lower the pH of the mash is covered in a later paragraph.
Preliminary water treatment
A simple water treatment which is a good basic treatment irrespective of the type of beer being brewed and irrespective of the water supply follows:
Table 3
Primary water treatment for 25 litres
Method
Put 25 litres of aerated water into your brewing boiler, add the 12g of calcium sulphate (2 heaped teaspoons) and bring to the boil. Boil vigorously for fifteen minutes to half an hour. When the boiling period is complete switch off the heat and wait for the precipitate to settle out. Then add the 2 g Magnesium sulphate (half teaspoon) and the 3g of common salt (one teaspoon).
The above treatment is a good starting point for all styles of beers. However, for milds and stouts the calcium sulphate is sometimes omitted. If no calcium sulphate is used in your water-treatment then use 4g (1 teaspoon) of flaked calcium chloride instead of the calcium sulphate.
Calcium carbonate is not completely insoluble in water but its solubility is very low. However, its solubility is reduced even further if there are other calcium ions present in the water. It is therefore beneficial to add any calcium sulphate used in your water treatment before the boil is begun. The addition of calcium chloride is often recommended in circumstances where calcium sulphate is not used. This is done to reduce the solubility of carbonate for the same reason. The magnesium sulphate is added last because it can retard the precipitation of carbonates. It dissolves easily.
The addition of 4g (one teaspoon) of citric acid to the water may also be useful as a matter of course, particularly in areas where the water is fairly alkaline. The use of acids for water treatment is covered in a later paragraph.
How will I know if my water is okay?
You will not know if your water treatment is correct until you brew with it. So it is really a chicken and egg situation. If you are already producing high quality ales that you are satisfied with, and which are the envy of your friends, then by definition, your water is fine. However, the primary reason for water treatment is to ensure that the mash conditions are correct. If after a trial brew without water treatment the pH of the mash is any higher than about 5.8 it may be desirable to perform some sort of water treatment and try to get it closer to pH 53.
Indicator papers for measuring pH are available from home brew shops. I prefer the narrow-range ones that are manufactured by Johnsons of Hendon. Simply damp a strip of the paper by dunking the tip of it into the mash, and compare its colour change with the colour chart provided for best results compare the colours against a white background; a white saucer or tile suffices.
Low cost electronic pH meters are available from laboratory equipment suppliers.
Fine Tuning
If after a trial brew with this treatment you find that the pH of your mash is too high, then add more calcium sulphate when you next brew. If the pH is too low then add less calcium sulphate. Keep adequate records and eventually you will devise a water treatment that is appropriate for your needs.
However, mash reactions are self buffering to a great extent. This means that provided the water is supplied with an adequate supply of the appropriate minerals, the mash pH will sit exactly where it prefers to be. Minor or even relatively large adjustments to the mineral content of the water is unlikely to shift it much.
Grists that contain a large proportion of roasted malts will be naturally more acidic than those that do not, but then darker beers are traditionally mashed at a lower pH, so the shift in pH, if any, is in the right direction. But, in any case, roasted malts are not used in very high quantities in modern beers.
Different types of beer may require different water treatments, but these differences are more to do with changes in perceived flavour than any scientific benefit. Sulphates are considered to produce a dry flavour most suitable for pale ales, whereas chlorides supposedly bring out the fullness of flavour which is required for darker beers. A high sulphate content along with a high chloride content can give a harsh flavour, therefore these flavour adjustments are performed by jiggling with the respective ratios of calcium sulphate and sodium chloride.
The amount of sodium chloride added is is a matter of taste. It has very little effect upon the mash reactions or any other aspect of brewing. It is said that dark ales and milds benefit from a higher quantity of salt. Typical quantities vary from about zero to half a teaspoon for pale ale, and two and a half teaspoons for a stout, per 23 litre batch. But no hard and fast rules can be applied.
There is no real point in adjusting the magnesium sulphate content to be much different to that specified. The magnesium is a co-factor, or co-enzyme for the yeast, and the water only needs to contain an adequate supply of magnesium ions; half a teaspoon is ample.
For persons of a more experimental turn of mind, table 6.4 shows the most important compounds found in, or added to, our water and their breakdown expressed as a percentage of their weight. As an example; 100 mg of calcium sulphate added to a quantity of water will add 29 mg of calcium and 75mg of sulphate. If the same quantity is added to 1 litre of water it will increase the calcium content by 29 ppm and the sulphate content 75 ppm.
Table 4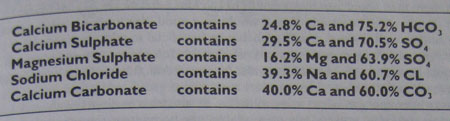
Adding minerals directly to the mash
It is not absolutely necessary to add the minerals to our brewing water. It is quite possible, and perfectly acceptable, to mix all or part of them in with our dry grist prior to mashing. In fact, in certain circumstances it may be beneficial to do so. Calcium sulphate is difficult to get into solution in the boiler, whereas the problem does not exist if it is mixed into the mash. Likewise, all of the magnesium sulphate and sodium chloride (salt) can be added to the mash without penalty. Of course, the water will still need to be boiled to remove calcium bicarbonate in hard water areas, and it will be desirable, to add some calcium sulphate or calcium chloride to reduce the solubility of the carbonate when this boiling process is going on.
It is also a useful method of making emergency adjustments to the mash pH, or for making immediate adjustments for experimental purposes. Calcium sulphate can be added to the mash to lower the pH or calcium carbonate (precipitated chalk) added to raise the pH. Immediate changes in pH will be achieved if the mineral is mixed well in. Calcium carbonate can be added to the mash to raise pH, were it will react with the other mash constituents, but it cannot be added to the water because it is insoluble and will settle out again. Anyway, it would be rare for anyone to want to raise mash pH, so this is mostly academic. Citric acid or lactic acid can also be added directly to the mash or to the water to lower the pH. The use of acids is described later.
Calcium conundrum!
There is one important little point that often puzzles people. Calcium sulphate is alkaline and actually increases the pH of the water to which it is added, but because it reacts with phosphates in the mash, releasing phosphoric acid, it reduces the pH of the resulting mash after the water has been introduced to the grain. It is important not to confuse the pH of the brewing water with the pH of the mash. They are not the same thing and confuse matters by moving in opposite directions when calcium sulphate is added. It is the pH of the mash that we are mostly interested in, after the water and grain have been mixed; do not concern yourself unduly about the pH of the brewing water prior to mashing.
Citric acid and lactic acid
An effective way of reducing mash pH is to add citric acid or lactic acid to the brewing water or to the mash. It is an easier and more sure-fire method of adjusting mash pH than the use of min-erals; many commercial breweries treat their water with acid. In some areas, where the water is very alkaline, it may, in fact, be necessary to add these in order to lower the mash pH to the desired figure. Calcium sulphate can be difficult to get into solution, and with some types of water a buffering effect takes place. That is, the addition of sulphate will move the pH a certain degree and then it will stubbornly sit still, irrespective of how much additional sulphate is added. Acids are more persuasive and have the additional benefit of forcing more carbonate to precipitate. If more than about four teaspoonful of calcium sulphate are required for a 25 litre batch of beer during water treatment, then it would be a good idea to give a helping hand by adding some acid as well. Indeed, it will probably be beneficial to add a teaspoonful of citric acid as part of the preliminary water treatment gjven in table 3 - just as a matter of course. The citric acid should be added at the end of the water filing period. Alternatively, it can be added directly to the mash and stirred in.
The same principles apply as when using minerals, ie, aim for a mash pH of about 5.3. Both citric acid crystals and lactic acid crystals are available from home-brew shops and chemists.
Water filters
Relatively inexpensive through-flow water filters are available which will attach to the tap and remove chlorine, heavy metals and other impurities from the water supply. They usually contain a silver-impregnated, activated carbon cartridge which removes certain impurities but allows important minerals to pass through. These may be useful for some brewers, particularly kit brewers who live in an area where the water is heavily chlorinated, and who do not wish to boil their water to remove chlorine. Some home-brew shops sell these filters. They do not remove calcium bicarbonate and are not a substitute for normal water treatment.
Domestic water softeners are fairly commonplace these days. The cheaper water softeners work by exchanging calcium ions with sodium ions, but sodium ions end up in the water instead of calcium ones. The sodium ions do not clog pipes or cause soap to be inefficient, but will interfere with brewing. The water from these softeners tastes "salty" and these are not suitable for brewing purposes.
True water de-ionisers are increasingly being fitted in domestic water supplies. These, unlike the simple water softeners mentioned above, remove both calcium bicarbonate and calcium sulphate and produce completely soft water. The output from these is suitable for brewing but, because they remove beneficial minerals as well, the water may need to be hardened, or Burtonised, by adding calcium sulphate to bring mash PH to its intended level.
Blowing a myth
Almost all home-brewing articles published to date instruct the home brewer to add precipitate of chalk to his water when making dark ales and stouts. Apart from the fact that no ale will benefit from indiscriminately chucking handfuls of carbonate into the water, precipitate of chalk will immediately precipitate out again! Calcium carbonate (chalk) is, as near as ninepence, insoluble in water. This, as another home-brewing author quite rightly states, is why the White Cliffs of Dover are still there after umpteen million years! A perfect analogy. I wish I had thought of it myself.
Read also:
Ingredients for brewing: grain, sugar, malt extract
Traditional Commercial Brewing
Home Brewing. Beginners Start Here
Home Brewing. What should You Know about the Hops

