 At first glance at this article it may seem that mashing is an extremely difficult process and super-critical as regards temperature, time, and 1001 other things. Please rest assured that it is not. The mash is surprisingly easy, tolerant, recovers well from up-cocks, and rarely fails. It is time consuming - yes, but it is not difficult or particularly critical. If you do not understand some of the technical aspects of this chapter do not let it concern you. You can quite safely skip the difficult bits. You do not really need to know what goes on during the mash, just as you do not need to know the chemical reactions that take place when baking a cake. Mashing is similar to making a pot of tea, except that the temperature at which the infusion takes place is lower.
At first glance at this article it may seem that mashing is an extremely difficult process and super-critical as regards temperature, time, and 1001 other things. Please rest assured that it is not. The mash is surprisingly easy, tolerant, recovers well from up-cocks, and rarely fails. It is time consuming - yes, but it is not difficult or particularly critical. If you do not understand some of the technical aspects of this chapter do not let it concern you. You can quite safely skip the difficult bits. You do not really need to know what goes on during the mash, just as you do not need to know the chemical reactions that take place when baking a cake. Mashing is similar to making a pot of tea, except that the temperature at which the infusion takes place is lower.
Read also:
Ingredients for brewing: grain, sugar, malt extract
Traditional Commercial Brewing
Home Brewing. Beginners Start Here
Home Brewing. What should You Know about the Hops
All You should Know about Brewery Yeast
Home Brewing. Water and Water Treatment
In general, if you mash at a temperature maintained somewhere between 62°C and 68°C, and sparge with water at 77°C to 80°C, you will get good results. Do not concern yourself unduly with the subject of mash pH, you will get acceptable results irrespective of what your actual pH might be; this sort of stuff is for perfectionist enthusiasts.
Introduction
Mashing is the process whereby the fermentable sugars are extracted from our malt grain and adjuncts. In simple terms, the mash consists of steeping our grain in hot water at a carefully maintained temperature (of about 65°C) and allowing it to stand for up to two hours. During this standing period, the enzymes produced by the pale malt convert the starch contained in the pale malt and in the other ingredients i nto various types of sugar.
Sparging is brewerspeak for rinsing the goodness out of the grains. After the mashing period has elapsed the sweet wort is drained from the mash bed and then the grains are rinsed with hot water of a temperature of about 77°C.
Wots'n enzyme?
An enzyme is the biological form of a catalyst. Its presence is required as a link in the chain of events that comprise a particular biological action. Enzymes are an important part of any bio-logical process, and are utilised by plants and animals to perform particular functions. Different enzymes perform different functions. During mashing, enzymes convert starch, which is a complex sugar, into simple sugars. Most of these sugars will be fermentable by the yeast; but some will be non-fermentable.
In the true sense of the word, an enzyme, as in a catalyst, is not actually consumed during the particular biological process. When the particular process that the enzyme performs is complete, it is released to do more work elsewhere. However, in mashing, certain enzymes are forced to operate at the top of their temperature tolerance, which has the effect of giving them a short active life before they are destroyed by the heat. This is not normally a problem because replacement enzymes are usually continually unlocked as part of the process being performed.
There are a number of enzymes at work during our mashing process and they are known collectively by the old-fashioned term of diaste complex enzymes. There are two diastatic enzymes in which we are particularly interested. The first of these is called alpha-amylase, which converts the starch in our malt into dextrins. The second enzyme is called beta-amylase, which converts starch and some of the dextrinous products of alpha-amylase into maltose. Maltose is a sugar that is fermentable by our yeast, wheareas dextrin is a group of sugars which are non-fermentable by our yeast and remain in the finished beer to provide residual sweetness and body. Alpha-amylase is sometimes called the dextrinogenic enzyme, and beta-amylase the saccharinogenic enzyme.
Mash temperature and pH
All enzymes are very fussy about the conditions under which they will work. They are particularly sensitive to temperature and acidity (pH). Alpha-amylase prefers a temperature of about 70°C and a pH of about 5.6, whereas beta-amylase prefers a temperature of about 60°C and a pH of about 5.0. Mash temperatures range from 60°C to 70°C, with 65°C being typical, and mash pH ranges from about pH 5 to pH 5.6, but pH 5.3 is typical.
At lower temperatures than their optimum, both enzymes still perform, but at a slower rate, which is in accord with most chemical and biological processes. However, at higher temperatures than their optimum the enzymes become progressively deactivated by the heat. Alpha-amylase begins to become deactivated at temperatures above about 70°C, and beta-amylase begins to become deactivated at temperatures above about 62°C. At increasing temperatures above these the enzyme's life is progressively reduced until, at some particular high temperature, the enzyme is destroyed immediately it is produced and cannot perform any useful work.
As for pH sensitivity, the enzymes are not destroyed or deactivated over the range of pH values likely to be encountered in our mashing process, but they work faster and more efficiently at their optimum value.
It is usual for British commercial and home brewers to mash at a single temperature. Therefore, for best mash efficiency we want both enzymes to work in conjunction with each other at the highest collective efficiency we can achieve. This means a compromise must be found between the optimum temperature and pH for both alpha amylase and beta amylase. Obviously, a point midway between the optimums will give maximum benefit to both enzymes. It therefore follows that a temperature of 65°C and a pH of 5.3 will give us maximum mash efficiency At these figures the mash reactions work fastest and maximum extract is achieved. This is sometimes referred to as maximum diastatic activity.
AS you will by now be aware, the PH and temperature of the mash has an important bearing upon mash reactors. The relative speed at which the two important enzymes operate is affected to a large extent by differing PH and temperature values. There are a number of other important reactions taking place during our mash alongside the conversion of starch into sugars, some of these reactions are due to enzymes, others are due to other biological or chemical processes. All contribute to the flavour and stability of the finished beer in one way or another, and all are affected by temperature and pH.
Mash products
The reason for mashing is to extract sugars from our grain. The sugars extracted from our malt fall into two types: maltose which is 100 per cent fermentable, and dextrins which consist of s0me non-fermentable sugars and some very slowly fermentable sugars. There are also other bits and bobs extracted which I have termed residual solids.
Maltose is 100 per cent fermentable. It is converted into glucose by the yeast during fermentation and yields alcohol in the finished beer. Maltose is used up during primary fermentation, and ferments into alcohol within a few days. It is therefore regarded as a fast fermenting sugar.
Dextrins are mostly non-fermentable, but also contain some very slowly fermentable sugars. These slowly fermentable sugars are attacked during maturation, and provide secondary fermentation and condition. Yeast continues to attack dextrins for a great many months, even years, and are therefore regarded as slowly fermenting sugars. Dextrins are responsible for much of the flavour and mouth-feel of beers.
Table 1 shows typical percentages of the respective components.
Table 1
Approximate constituents of a typical wort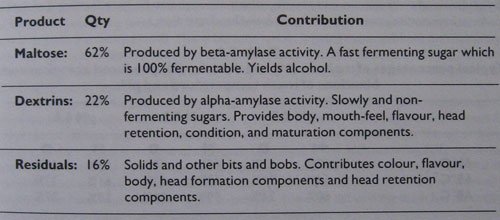
Control of Fermentability
An important aspect of mashing, as opposed to brewing from malt extract, is that we have control over the ratio of fermentable to non-fermentable sugars in our wort, ie the fermentability. A wort containing a high proportion of non-fermentable dextrins would be of low fermentability and would yield less alcohol than a high fermentability wort of the same gravity, but the beer would have more body, flavour, and mouth-feel. The opposite is true of a wort which contains a low proportion of dextrins.
To recap upon what we know so far: An enzyme, alpha-amylase, converts the starch in our malt into a group of non-fermentable sugars known as dextrins, then (or alongside this) another enzyme; beta-amylase, converts starch and dextrins into a fermentable sugar known as maltose. We also know that alpha-amylase is quite happy to work over a temperature range of 60°C to 70°C, but that it works slowest at 60°C and low pH levels, and that it works fastest at 70°C and high pH levels. We also know that beta-amylase works fastest at around 60°C and at low pH values, but is progressively deactivated at temperatures above this. It should now be apparent that by messing around with temperature and pH, we are able to control the speed at which one enzyme works relative to the other, and thereby control the ratio of maltose to dextrin in our wort.
It then follows that if we are able to slow down the activity of beta-amylase relative to alpha-amylase, and then stop the mash before some of the dextrins are converted to maltose, we would have a wort containing a high proportion of non-fermentable sugars, resulting in a full-tasting beer after fermentation.
Conversely if we provided optimum conditions for beta-amylase activity, slowed down the alpha-amylase activity, and permitted ample time for as much of the dextrin as possible to be converted into maltose, we would have a dry beer. However, in practice, we will always have a certain degree of residual sweetness remaining in our wort, because not all of the dextrins produced by the alpha-amylase are open to attack by beta-amylase. Furthermore, apart from supplying residual sweetness, dextrins also supply body, mouth-feel, and flavour to the finished beer. A beer that is brewed to be very dry could be of a rather thin taste.
The range of mash pH over which acceptable results will be obtained ranges from about pH 4.5 to about pH 5.6, where pH 4.5 tends to produce a dry beer, and pH 5.6 tends to produce a sweet beer. It is normal British brewing practice to provide a mash pH of about 5.3 and to control fermentability by means of the mash temperature; only lowering the pH if a drier beer is required than can be obtained from temperature adjustments. If a very dry beer is required it may be necessary lower the pH to a value close to pH 4, and it may be necessary to add lactic acid to the mash to achieve this value. High mash temperatures favour the extraction of high molecular weight nitrogenous compounds, which although can be haze forming if present in too large a proportion, provide good flavours and head retention.
Table 2
Typical percentages of maltose (M) and dextrins (D) produced at various combinations of mash temperature and pH.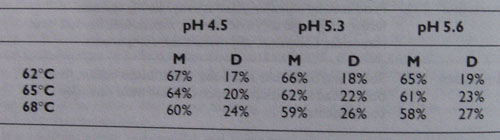
The general trend of table 2 should be observed. The figures should only be taken as approximate because so many other conditions affect the actual results. Apart from temperature and pH, factors such as mash time and mash stiffness also affect the results. Indeed, maximum efficiency occurs at 65 C and pH 5.3, which is sometimes more important than the actual sugar ratio; particularly to commercial brewers.
The sensitivity of amylases to changes in temperature is so great that, in Britain, the working range of mashing temperature is limited to within a few degrees above or below 65°C. Such small changes have a powerful effect on the flavour, rate of conditioning, and other properties in the finished beer. Quick maturing beers, intended for quick consumption, are usually mashed at the lower end of the temperature range; whereas strong, long maturing beers are usually mashed at the higher end of the temperature range.
A lower temperature, say 63°C, is often used for brewing mild ales; a middle temperature, say 65°C is suitable for pale ales; while a high temperature, say 67°C, is often used for strong beers and stock ales.
The higher temperatures produce a higher proportion of slowly fermenting dextrins and therefore give the yeast something to chew on while the beer is maturing and produce characteristic flavours as a result. Beers brewed at the lower temperatures contain less dextrins and are there-fore ready sooner. It is worth bearing in mind that a low mash temperature slows down the overall mash reactions to a certain degree and this contributes to the longer mash times sometimes advocated for drier beers.
It is possible to produce a very high fermentablity wort, say 70 per cent maltose, by mashing at two temperatures, each temperature being the optimum for the two important enzymes, namely: 60°C and 70°C. Many of the drier lagers are produced in this way. Some commercial British breweries that wish to produce a fairly dry beer begin the mash at 62°C, but about half way through they raise the temperature to 68°C by letting in hot water, and the mash is completed at this higher temperature.
Mash times
Another aspect of control is mash time. British infusion mash times typically vary from about one-and-a-half hours to about two-and-a-half hours, depending upon the type of beer being produced. During the latter part of the mash all of the starch has been converted to maltose and dextrin, and the beta-amylase is engaged in converting the dextrins into maltose. Given a sufficiently long mash time the maximium amount of dextrin would be converted to maltose, but this is, of course, undesirable if we are producing a sweetish beer. It is, therefore, obvious that the mash should be stopped as soon as the desired point has been reached. In fact, irrespective of the type of beer being produced, it is desirable to keep the mash time to the minimum necessary to achieve the desired result. This is because tannin is being extracted from the malt alongside our sugar conversion. An excess of tannin will produce a bitter off-taste in our ale, similar to that of stewed tea. Over-mashing, and more importantly over-sparging, may extract an undesirable level of tannin from the goods in the mash.
I do not agree with the practice of overnight mashing as advocated by some members of the home-brewing fraternity. Apart from producing a particularly dry beer, this practice favours the extraction of tannins and other undesirable compounds from the malt. On the other hand, under-mashing can result in some of the starch contained in the malt not being fully converted, and this will result in a cloudy beer being produced. Fortunately there is a simple test that we can perform on our mash, namely the iodine starch end-point test, which will tell us when all of the starch has been converted into sugar. It does not, however, tell us if the mash has proceeded far enough for a suitable maltose/dextrin ratio to be achieved.
Starch end-point test
The starch end-point test can be used to determine when all of the starch has been converted to sugar. It is fairly straightforward. Place a small amount of sweet wort from the mash on to a white dish or saucer then, using a dropper, place a drop of tincture of iodine on to the wort. If the iodine immediately turns a deep blue-black, starch is still present, if not, it isn't-simple.
If a dry beer is being produced, a sufficient length of time should be allowed after starch end-point to ensure that all of the convertible dextrin has been converted into maltose. If a sweet beer is being produced, the mash should be stopped fairly soon after starch end-point has been reached. An average mash time is about one and a half hours, although the starch end-point may well have been reached within 20 minutes.
Mash stiffness
There is another aspect of the mash which, surprisingly, has an effect upon the fermentability of our wort, and that is mash stiffness, ie, the ratio of grist to liquor. Thinner mashes have the effect of producing a wort of higher fermentability than does a stiffer (thicker) mash. However, the effect of this parameter is so small with respect to the others that for our practical purposes we can ignore it.
Typical grist to liquor ratios can vary from about 1.5 litres per kilogram to about 3.5 litres per kilogrram without much difference being experienced in extract or residual sweetness. Lagers sometimes employ grist to liquor ratios of up to 5 litres per kilogram. In general we aim for a figure somewhere in the middle of the range, being about 2 to 2.5 litres per kilogram, or about 1.6 pints of water per pound of grain in yesterspeak. This means that a typical bitter using about 4Kg of malt will require 8 to 10 litres of mash liquor.
Stopping the mash
When a very sweet beer is being produced it may be considered desirable to stop the mash when the desired mash time has been reached, otherwise the conversion of dextrins into maltose can continue past the desired point, thereby producing a drier beer than was intended. This is particularly important if a long period of time is likely to elapse between the completion of the mash and the boiling phase. Bearing in mind that the run-off and sparging operation after the mash can take up to two hours, it can be seen that a considerable amount of additional conversion can take place during this period.
Stopping the mash simply entails rapidly raising the temperature of the mash to about 75°C, which has the effect of disabling the beta-amylase, thereby preventing any further conversion of dextrin into maltose, but it still enables the alpha-amylase to continue the conversion of any residual starches into dextrin, which otherwise could cause a haze to be formed in the finished beer.
Another reason for raising the temperature of the mash is to assist liquifaction by reducing the wort viscosity and making mash tun run-off easier. The classic way of stopping the mash is to add hot water to the mash until the desired temperature has been reached, but with home-brewing equipment this runs the danger of diluting the wort too much, causing the boiler to be too full after sparging. However, the practice of stopping the mash can be considered advanced home brewing, and in any case, stopping the mash by these means can be regarded as unnecessary for most beers. Sparging with water maintained at about 77°C and collecting the sweet wort in the brewing boiler set to maintain the same temperature is sufficient.
Mash efficiency
For every kilogram of grain employed in the grist, we would expect to achieve a particular extract. For instance, pale malt has a typical laboratory extract of 297 degrees per kilogram litre. This means that, under laboratory conditions, the recovered wort from a small mash containing 1 kilogram of pale malt would have a maximium specific gravity of 1.297, or 297 degrees per litre. If the recovered volume of wort was five litres the original gravity of the wort would be 297/5 - about 1059, but this assumes that we have 100 Per cent mashing efficiency. However, mashing efficiency is dependent upon the tyPe of mash tun, the volume of grist employed, and the method of sparging used. The laboratory figures are never achieved in practice. Many commercial breweries only achieve mash efficiencies of about 85 per cent, and we should not expect any better. Typically we will achieve about 85 per cent efficiency with ordinary strength ales, and, about 80 per cent efficiency with strong ales. As a safety measure, all of the recipes assume a mash efficiency of 75 per cent which is about the lowest likely to be experienced.
Maximum possible mashed extract
The strongest 100 per cent mashed brew, that is a brew with no copper sugars or syrups, that we are able to produce is governed by the size of the equipment available to us. Home-brewing equipment is based upon a brew length of 5 gallons or 23 litres. Most of the equipment available to us- brew bins, boilers and mash tuns - are based upon 25 litre vessels and a 25 litre brew bin, after allowing room for the yeast head, might hold 23 litres at best.
A mash tun based upon a fermenting bin could hold about 7.5 kg of malt along with the minimum quantity of about 2 litres per kilogram of liquor. At 80 per cent mashing efficiency we could expect a beer of about 1079 original gravity. However, at this concentration, sparging becomes difficult, and we end up with more than 23 litres of wort after we have sparged down to 1005. This means that not only would a double boil be required but prolonged boiling time would be necessary in order to reduce the volume back to 23 litres.
In my experience the absolute maximum gravity that we can expect to extract, keep good sparging efficiency, and end up with a manageable volume of collected wort is about 1065, and that needs care. If we wish to brew stronger ales than this, then we will need to add sugars or syrups to our boil.
Another way of increasing maximum gravity is not to sparge. The brewers of yesteryear did not sparge their wort in quite the same way as we do now. Sparging was not introduced until the early part of the nineteenth century. They mashed much larger quantities of malt per batch, twice or three times as much, and mashed the same batch up to three times; or at least, they thought that they were mashing three times.
The first mashing made the strong old nectars of perhaps O.G. 1100, the second mashing made common ale of perhaps 1075, and the third mash made table beer (T beer) or small beer of about 1050. Sometimes the first and second mashes were combined to brew a strong ale of about O.G. 1090. However, the second and third mashes were not real mashes at all, merely rinses.
Strike heat
The starting temperature of the mash is usually around 66°C (called initial heat). However, the mixing of the cold grist with the liquor lowers the temperature of the liquor somewhat. In order to compensate for this temperature drop, the liquor is heated to a temperature a few degrees higher than initial heat prior to the grist being added, such that when the grist is added the temperature of the mash falls to the initial heat value (around 65°C). This higher temperature is known as strike heat and is usually about 72°C. The optimum value of strike heat is dependant upon the grist-to-liquor ratio, the temperature of the grist, and the temperature of the air. It is best determined by experiment.
If the strike heat is too high some of the conversion enzymes in the malt may be denatured, and yet if it is too low the temperature of the mash may fall too far below the desired initial heat and an inefficient mash may result.
Retorrefication
Obiviously, the temperature difference between strike heat and initial heat is important. The closer strike heat is to initial heat the better. Anything that we are able to do to reduce this temperature difference is to our advantage. One technique for reducing the temperature difference is that of warming the grist prior to mashing, a technique known as retorrefication.
Fortunately it is fairly easy for us to utilise this technique. All we need to do is to put our grist into a dry, 12.5 litre brewing bin with a tight close fitting lid, fit the lid and float it in our hot liquor tank as the mash liquor is being heated. Obviously, the temperature of our grist will be close to the temperature of our mash liquor, therefore the mash temperature will not drop so much when the grist is added to the mash liquor. The only precautions that need to be taken are to ensure that the grist remains dry during retorrefication. However, retorrefication is not an essential operation.
The mash
Prior to the mash the hot liquor tank is filled with treated brewing liquor and heated to a temperature of about 2°C or 3°C above strike heat. When this temperature has been attained, a quantity of liquor equivalent to about 2 litres per kilogram of grist is allowed to flow into the mash
tun. The lid is fitted to the mash tun and a few minutes is allowed to elapse to enable the temperature of the liquor in the tun to stabilise. When a sufficient length of time has elapsed a check on temperature is made to ensure that the temperature of the liquor is at strike heat about 72°C. Corrections to the temperature can be made by stirring in cupfuls of cold or boiling water as appropriate until the desired temperature has been attained.
When proper strike heat has been achieved the grist can be added. The grist should be added as quickly as possible stirring frequently, but care should be taken to ensure that the grist is not added so fast that it is likely to cake into dry clumps. A gradual sprinkling motion is required, not a resounding splosh.
When all of the grist has been introduced to the tun it should be thoroughly stirred to ensure that there are no dry pockets and a check on temperature should be made to ensure that it is at the desired initial heat. If it is not, it should be adjusted as quickly as possible by adding boiling or cold water, while stirring, until the temperature is correct. Temperature can be quickly equalised throughout the mash by drawing some sweet wort into a jug, via the bottom tap, and pouring this back on to the surface of the mash. A wooden spoon fixed into the chuck of a modern, variable-speed electric drill makes an excellent stirring widget, but water and electrical equipment are dangerous combinations, so such things should not be attempted unless you are aware of the hazards involved.
If an adjustment to initial heat is necessary, then a note should be made of the error and for future brews the strike heat should be adjusted accordingly to eliminate the need for further adjustments at this stage. The lid should be fitted to the mash tun and thermal insulation placed over the lid. It should then be left to stand for the appropriate time of about one-and-a-half hours.
Temperature checks should be made about every half hour, but once you know the temperature characteristics of your mashing system, you should refrain from continually removing the lid of the tun and peering inside, otherwise too much heat may be lost in the process.
Adjustments to mash pH
Careful control of mash conditions will provide us with dependable, predictable, and repeatable results, but it is very rare for a mash to fail completely because of incorrect mash conditions. The process is surprisingly tolerant and recovers well after corrective measures have been taken. Ensuring the mash temperate is correct is of Primary importance during mashing; control of mash pH should assume secondary importance. Mash pH is controlled to a large extent by the water treatment prior to brewing, and correct water treatment should automatically ensure that the mash pH is correct.
The first time a particular beer is brewed it is desirable to ensure that the pH of the mash is at the chosen value. After the temperature corrections have been made to the mash, and it has been allowed to stand for about five minutes, a sample of the wort should be drawn off and the pH measured and noted.
The pH of the mash can be corrected by adding, directly to the mash, calcium carbonate if the pH is too low, or by adding calcium sulphate, (or lactic acid, or citric acid) if the pH is too high.
The salts should be added, a teaspoonful at a time, stirring thoroughly until the desired pH is reached. A note of the quantity of added salts should be made, and for future brews the water should be treated appropriately.
Calcium carbonate can be added to the mash to increase pH, but it is insoluble in water and should not be added directly to the water at the water-treatment stage because it will simply sink to the bottom of the boiler or other water treatment vessel and stare at you blankly. However, it is very unlikely that anyone would wish to increase mash pH.
In general if the pH of the mash is too high there is either too much calcium bicarbonate in the water or insufficient calcium sulphate. If the pH is too low there is probably too much calcium sulphate in the water.
Once the correct water treatment has been determined for a particular type of beer, then it is fair to assume that the water treatment will be the same each time a similar beer is brewed and that our mash pH will automatically be correct.
Running off
When the appropriate mashing period has elapsed it is obviously necessary to transfer the sweet wort from the mash tun into the boiler in readiness for the boiling stage of our beer production.
Running-off is accomplished by simply opening the tap in the mash tun and allowing the sweet wort to run gently into the boiler. The word gently must be emphasised because during the running off period, the grains in the mash tun settle down on to the false bottom and form a filter bed which serves to remove solid particles and debris from the wort. If the sweet wort is allowed to run off too fast, the filter action may be impaired, the grain may pack down and block causing a set mash, or debris may be drawn towards the tap and block that.
The first runnings from the mash are likely to be turbid, and these should be returned to the mash tun until the sweet wort runs bright, after which time the sweet wort can be allowed to run into the boiler.
As soon as the boiler element is covered with wort, the heater should be turned on and the thermostat/simmerstat set to 77°C This reduces the possibility of saccharification continuing in the collection vessel, but also it is desirable that the wort should not be allowed to cool before the boiling phase begins, which would favour the extraction of proteins and tannins and could cause off-tastes and hazes to be produced in the finished beer. When the sweet wort has been run off, the goods in the mash tun (by now known as grains) still retain much of the sugary matter and this must be flushed out - or in brewerspeak, the grains must be sparged.
Sparging
Sparging consists of gently spraying the spent grains in the mash tun with hot water maintained at a temperature of about 77°C. The higher temperature helps to prevent the extraction of undesirable substances from the grains. Sparging must be performed gently, otherwise the filter bed of grain could crack and allow debris into the wort. Sparging is usually performed by heating the water in the hot liquor tank to a temperature of 77-80°C and spraying the water over the grains by means of a length of flexible pipe with a watering rose at one end and with the other end of the pipe connected to the boiler tap. A spray ring can be constructed from copper tubing if desired. If such refinements are unavailable, the simplest method is simply to pour jugfuls of hot water over the grains taking care to ensure that the bed does not break up The specific gravity of the spargings is monitored and sparging is halted when this falls to below about 1005. Over-sparging should be avoided because it favours the extraction of undesirable substances from the grains which cause off-tastes and hazes, and affect head retention, apart from the risk of over diluting the wort. I must stress that sparging is a slow process; it must not be rushed. In commercial breweries the run-off and sparging operation can take two hours or more. If you break up the mash bed and end up with a turbid wort you may end up with a cloudy beer.
A common sparging technique, known as fly-mashing, does not drain the mash bed completely, but supplies sufficient sparge liquor to balance the out-flow from the mash tun and keep the mash bed floating. This technique is probably better if a set mash is likely; many commercial breweries use this method.
An equipment-free method. A simple method of rinsing the goodness from the grains, which does not require any special equipment is re-mashing; which is what the brewers of old did. When the first runnings from the mash have drained, the tap is closed and the mash tun is gently re-charged with water at about 80°C. It is then stirred thoroughly and left to stand for about fifteen minutes. After the standing period the mash tun is run off as before. Again return the turbid first runnings back to the mash tun for re-filtering. This operation can be repeated until the collection vessel is full, or the gravity is below 1005. This method does run the risk of pulling haze producing substances from the mash, but it has served generations of home brewers well without causing problems. I would recommend no more than two re-mashes; often just one will suffice. It is probably best to use boiled but otherwise untreated water for the second mash.
The decoction mash
The decoction mash is a mashing technique, traditionally used for the production of lager, which was developed by the Germans in the mid-nineteenth century in order to match the clarity of English pale ales. The British pale ale revolution in the mid-nineteenth century was a swing in public preference towards transparent beers. The clear beer brigade, the yuppies of the 1850's, drank the up-market pale ales. The Germans presumably experienced a similar swing in preference and no doubt faced competition from English pale ales.
British pale ale was made possible by Britain's indigenous breed of two-rowed barley, the climate, and superior malting skills. The Germans were forced to cope with high-nitrogen, poorly modified malts, made from indifferent breeds of barley. Thus the decoction system evolved. The Term "decoct" means to prepare by boiling. A decoction mash is started at low temperature and the temperature is gradually raised in a step-wise fashion by frequently removing a proportion of the mash, say a third, boiling it, and then returning it to the main mash. The main features of a decoction mash are a low temperature protein rest period at 45 C to 55 C, and the regular boiling of a fraction of the mash.
The protein rest period allows excessive protein, contained in high-nitrogen malts, to be degraded into simpler substances, a process known as proteolysis. The boiling of portions of the mash tackles another problem; it gelatinises the starch present in poorly modified malts which enables it to be attacked by various enzymes during the mash. Both protein and starch would cause hazes in the finished beer if they were not attended to. The traditional decoction mash requires the use of two vessels. The first of these is an insulated mash tun similar to traditional British brewing practice. The second is a smaller vessel, capable of being heated, similar to a copper, which we will term the mash "cooker". Methods, particularly timings, vary greatly, but a typical triple decoction process would proceed as follows:
The grist is mixed into the mash vessel with warm water such that the initial temperature of the mash is 35°C to 40°C The mash is then allowed to stand for anything from half an hour to two hours depending upon the brewery's preference. Some proteolysis takes place at this temperature, but the real reason for this standing period is to ensure the grist is well mixed and hydrated and that the enzymes are activated. Traditionally, as in British brewing practice, men would be continuously stirring the mash for this standing period.
After the standing period has elapsed, about a third of the mash, grain and all, is removed from the mash tun and transferred to the cooker. The temperature of the fraction in the cooker is raised to 65°C and held there for about half an hour to allow saccharification to take place. It is then raised to the boil. This is the first decoction.
The boiled portion of the mash is then added to the main mash and stirred in. This raises the temperature of the main mash to about 50°C; the optimum temperature for proteolysis. The mash is then allowed to stand for about an hour. This is the protein rest period.
After the protein rest period has elapsed, a portion of the mash is again transferred to the cooker, held at 65°C for about half an hour, and then raised to the boil. This is the second decoction.
The boiling portion of the mash is then added to the main mash and stirred in. This raises the temperature to about 65°C; the optimum for saccharification. The mash is allowed to stand for an hour or an hour-and-a-half. During this period starches are being converted to fermentable sugars.
After the standing period has elapsed the third decoction takes place. Again a third of the mash is transferred to the cooker and it is brought to the boil. The boiling mash is added to the main mash which raises the temperature to about 75°C. This is the optimum for temperature for starch liquefaction, which reduces wort viscosity and aids run off. It also deactivates the enzymes. The mash stands for about half an hour at 75°C and then it is transferred to another vessel known as a lauter tun, where the sweet wort is run off and the goods sparged in the ordinary manner.
The lauter tun is a separate vessel where the wort is separated from the grain and the grain sparged. This has a false bottom similar to a traditional British mash tun. The Germans were forced to use a separate vessel because the decoction mash involves a good deal of pumping the mash, grain and all, from the mash vessel to the mash cooker and back again. This would obviously be impossible if the main mash vessel had a false bottom holding the grain back, as in British mash tuns. Some of the larger British high-tech breweries use lauter tuns because of the increase in throughput they afford. By transferring the mash to a lauter tun for wort separation, the next brew can be mashing while run off and sparging is taking place.
The above remarks apply to the triple decoction mash. The double decoction process is very similar, except that the grist is mixed in at 50°C instead of 40°C. Two decoctions, or boils, are then employed to raise the temperature; first to 65°C and then to 75°C as described above. The decoction mash is technically redundant, although it is still used by some German breweries as a matter of tradition. The classic triple decoction mash is energy inefficient, requires three vessels (including lauter tun), can take up to six hours to perform, and is quite unnecessary in most cases. Many German breweries now use the more time and energy-efficient single temperature infusion mash, or temperature-stepped infusion mash, and many of those that still use the decoction method use simplified versions of it. Nobody uses under-modified malt these days, so the necessity to boil part of the malt to gelatinise the starch has long passed. Any maltster who supplied under-modified malt would willingly hang up his clogs and retire to Bournemouth in shame - even German maltsters! The protein rest period is also unnecessary in all quality beers, whether they be British, German, or anyone else's. All of the ingredients used in British beers are compatible with the single temperature infusion process; as are most of those of Europe (but not the world).
The home brewer can imitate the decoction mash if he so wishes. It is relatively easy to bale out a proportion of the mash with a saucepan and boil it, but I would not advise it. An important disadvantage with decoction mashing is that every time a third of the mash is boiled to raise the temperature, a third of the available enzymes are destroyed and saccharification becomes progressively less efficient. With modern, controlled-nitrogen, well-modified, brewing malts, the brewer will soon run out of enzymes and run into trouble. Remember that the decoction method was developed to cope with bad malt; you are, hopefully, not going to get bad malt!
However, with some types of beer it may be desirable to include a protein rest period in the mash cycle, but this is best performed using the temperature stepped infusion mash, described later. But first, I sense a discussion about proteolysis coming on!
The protein rest – proteolysis
The main purpose of the protein rest period is to reduce the haze potential in the finished beer. Beers made from top quality ingredients have little haze potential, so there is no need for a protein rest period for the vast majority of European style beers, including German lagers.
No English-style beer needs a protein rest period. We are very lucky in Britain, We have the best brewing barley in the world. Our farmers know how to produce high-quality, controlled-nitrogen, two-rowed brewing barley and our maltsters know how to malt it to perfection. I would I ike to think that this is the result of the accumulated wisdom of generations of men, but I suspect that our climate has a lot to do with it. So next time you feel like complaining about our weather, just think of the best brewing barley in the world.
The barley grown in some other countries, such as America, and Australia, is usually six-rowed barley and high in complex nitrogenous substances such as protein. An excess of these will give rise to hazes being formed in the finished beer and considerably reduce shelf-life. The malt made from such barley is well-modified, but is much higher in protein than is acceptable for brewing purposes. The brewers in these countries tend to dilute the nitrogen content of their beers by using very high quantities of cheap, locally available unmalted adjuncts such as rice grits, maize grits, or sorghum grits. Not only do the beers of these countries contain high amounts of haze-forming proteins and starches, but the barbarians also serve them freezing cold. Highly-chilled beers are much more likely to throw a haze. Beers of this style benefit from a protein rest period to enable the proteins in the malt to be broken down into simpler substances and for the starches in the adjuncts to be opened up (so to speak).
High-nitrogen malts contain an enzyme Proteinase which is capable of breaking down high-order proteins in the grist into amino acids and other low-order nitrogenous products, a process known as proteolysis. Another effect of this process is to render "bound" starch open to attack by amylases. Proteinase contained in the malt can be used to unlock starch contained in the malt can be used to unlock starch contained in unmalted cereal adjuncts, such as rice, oats, and maize.
Proteinase is sensitive to high temperatures and is only active between 45°C and 55°C. It is destroyed at temperatures much above this. The protein rest period is normally held at about 50°C to enable proteolysis to take place. Proteolysis does not take place at normal British infusion mash temperatures.
Proteinase is so temperature-sensitive that it can be destroyed at the time of malting. The traditional reason for so-called "lager malt" being very pale in colour, was because it was only lightly kilned in order to preserve the proteolytic enzyme.
Temperature-stepped infusion mash
For brews that require a protein rest period, a more acceptable alternative to the decoction mash is the temperature-stepped infusion mash, sometimes referred to as the temperature-programmed mash. As the ancient practice of boiling poorly modified malt to gelatinise the starch is no longer required, there is no point in doing so. There are better methods of raising the temperature of the mash after the protein rest period which does not destroy the important enzymes and reduce the enzymic activity of the mash. Modern malts are not particularly rich in enzymes, so it would be foolish to go around deliberately destroying them before they have done their work.
As mentioned earlier, the vast majority of beers will not benefit from a protein rest period; the single temperature infusion mash is perfectly adequate for most beers and lagers. Although it is probably true to say that a protein rest period will reduce the haze potential of any beer, it is simply that most quality beers have little inherent haze potential; so the problem does not arise. In Britain, at least, we ate unlikely to be supplied with high nitrogen malt unless someone is sneaking cattle-feed malt into our supplies, so we are unlikely to be bothered with excessively high protein levels.
The most likely beers to benefit are those that use a high proportion of starchy unmalted adjunucts, raw cereals, or are expected to be served highly chilled. A temperature stepped mash will be required in the event of a home brewer wishing to imitate American or Australian beers, and may also be appropriate if someone wishes to imitate the very dry "more of the sugar is turned to alcohol" type of lager. Wheat beers and one or two other unusual types may also benefit.
Table 3 shows the optimum temperatures for the various enzymic reactions which take place during a temperature stepped infusion mash. The actual temperatures employed often vary from the optimums, usually at a point somewhere between two optimums so that two enzymes are working in concert. Not all mashing methods have steps at ail of the temperatures shown. Methods vary according to the characteristics desired.
Observe that the 60°C, 65°C, and 70°C temperatures are similar to the single temperature infusion mash and the same rules apply.
Table 3
Optimum temperatures for mash reactions.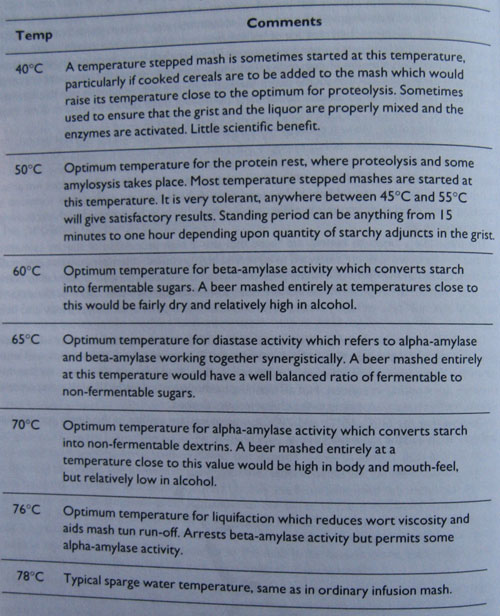
Mash programmes
Programme 1 is equivalent to the classic triple decoction mash which was developed by the Germans during the nineteenth century to cope with poorly modified malts, and high protein malts. It is still used by some breweries, particularly those that use a high level of unmalted cereals in their grist. The cereals should be cooked while the mash is standing at 40°C and added to the mash before th 50°C stage.
Programme 1
Equivalent to triple decoction
Programme 2 is equivalent to a double decoction mash. It is a simplified version of the triple decoction mash. The mash is speeded up by eliminating the 40°C stage, which is only necessary if separately cooked cereals are to be added to the mash, whereupon the temperature of the mash will be raised to the more important temperature of 50°C. If cooked cereals are not being added, the mash can be started at 50°C as in this example.
Programme 2
Equivalent to double decoction
Programme 3 is equivalent to the single decoction mash, and is the temperature stepped mash reduced to its simplest form. It is a further simplification of the above programmes by eliminating the liquifaction stage at 76°C. The liquifaction stage only makes wort run off easier and arrests the enzymic activity. It is not strictly necessary and is not done in the British infusion mash process. Many German breweries have moved to the single decoction because it saves the energy required to raise the temperature, and the time taken in doing so.
Programme 3
Equivalent to single decoction
Programme 4 shows a typical variation for very dry beers of high fermentability. It features an additional low temperature (60°C) saccharification stage to increase fermentability. The 76°C stage can be omitted if required.
Programme 4
Modified programme for very dry lagers
Programme 5 shows a mashing programme given to me by a Dutch home-brewer as part of a recipe for a wheat beer. It is included to demonstrate how complicated these things can be when taken seriously enough. Some of these steps are quite unnecessary for the recipe supplied (50 per cent lager malt, 40 per cent wheat malt, 10 per sent flaked oats), but it would produce a characteristically light dry beer for those wishing to attempt such things.
Programme 5
Seven step infusion mash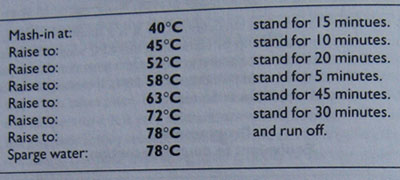
Raising the temperature
In a simple temperature stepped mash, where the temperature is raised only once or twice, the temperature can be raised by adding boiling water and stirring it into the mash quickly. The fact that the additional water thins out the mash is not a problem; stepped mashes are usually on the thin side anyway. The boiled water should be treated water if you normally use water treatment. For anything more than about two temperature steps a heated mash tun will be necessary due to a lack of space in the mash tun. The boiler and grain bag method can be used, or a heated mash tun as described in the chapter on equipment. The optimum rate of temperature rise for a heated tun is about 1°C per minute. The temperature should be regularly equalised by drawing wort from the tap on the mash tun, using a jug and sprinkl ing it over the surface of the mash.
Cereal cooking
Some countries make extensive use of raw cereals in their beers, usually in the form of grits. America and Australia, for instance, tend to use maize, rice, and sorghum grits quite heavily. Many other countries use one or more of these. Grits are cereals that have had the bran and embryo stripped away by milling and they need to have their starch gelatinised by cooking before they can be used in the mash tun.
The cooking of these cereals simply entails simmering them in water for fifteen to twenty minutes before adding them to the mash tun. Both the cooked cereal and the water they were boiled in should be added to the mash tun.
The most likely grit that a home brewer might wish to use is rice. Domestic rice should be broken into a smaller particle size with a rolling pin before cooking. The flaked and torrefied cereals that are typically used in British brewing practice do not need cooking. The starch in these has already been gelatinised by heat treatment at the factory.
Wheat is a special case. Malted wheat, flaked wheat, and torrefied wheat do not need cooking. However, some Belgian wheat beers tend to use high proportions of raw, unmalted wheat grain in their make up and this is a little more complicated. The gelatinisation temperature of wheat is about 60°C and will perform well at the normal saccharification temperatures ised in British brewing practice without cooking. However, if the beer requires a protein rest period at 50°C, then the wheat grain will need to be cooked first. In some cases it may be easier to substitute torrefied wheat or flaked wheat for the raw wheat, and the cooking stage will be eliminated.
If cooked cereals are being used in a recipe, it is advisable to start the main mash at 40°C and hold it there while the cereals are being cooked. When the boiled cereals and the water they were boiled in are added to the main mash, the temperature of the main mash will rise somewhere close to the protein rest temperature.
Read also:
Ingredients for brewing: grain, sugar, malt extract
Traditional Commercial Brewing
Home Brewing. Beginners Start Here
Home Brewing. What should You Know about the Hops

